The Truenat Beta Cov/ SARS Cov-2 are Chip-based real-time PCR tests. It is used in the diagnosis of COVID-19. The step-by-step procedure ( with Pictures), interpretation of results (5 variants of reporting), and the advantages and disadvantages are all mentioned below.
Keep on reading…….
The ICMR (Indian Council of Medical Research) recommended using the Truenat machine, specifically BetaCoV, for screening tests for COVID-19 on 4th April 2020.
It was also approved using two machines that are US-FDA approved and based on real-time Polymerase Chain Reaction (RT-PCR). They are GeneXpert and Roche Cobas- 6800/800. It is only approved for emergencies.
Also, ICMR mentioned using Biosafety cabinets of levels 2 and 3 with all biosafety precautions to use the Truenat Beta CoV test.
Truenat machine is a portable, battery-based- operated PCR analyzer. It also requires minimal training, unlike open system RT PCR.
The cost of Truenat machine ranges between 4.5 lakhs and 12 lakhs and received the approval of the Government of India and ICMR both.
Principle Of The Truenat Test:
Truenat Beta CoV works on Real-Time Reverse Transcription Polymerase Chain Reaction (RT PCR) principle based on Taqman chemistry.
PCR amplified DNA and makes lots and lots of copies of DNA. Using the PCR method, the smallest amount of DNA can be amplified in order to analyze it. For example, it can be used to Amplify even the smallest trace of an infectious virus or bacteria from a biological specimen.
The process involves finding a specific piece of DNA in a sample using an enzyme called DNA polymerase and periodic cycling of temperature, which anneal and splits standards, respectively. With each round of annealing and splitting, the number of nucleic acid doubles leading to exponential amplification of the starting trace nucleic acid in a short amount of time. The amplified amount of DNA is then analyzed separately for identification.
Real-time PCR is a variant of the standard PCR method. It has several advantages over regular PCR that has been made at the PCR method of choice.
Real-time polymerase Chain Reaction involves simultaneously amplifying and detecting the target DNA during every cycle, thus generating accurate information about the presence of DNA and its quantity in each reaction, end therefore removing the unnecessary need of the post-PCR product analysis, separately.
The Truenat Duo Real-Time Quantitative micro–PCR analyzer is a Real-Time PCR-based system.
Preferred Sample: Oropharyngeal and Nasopharyngeal Swab
Screening test for Beta Cov Chip Test: Semi quantitive detection of Beta CoronaVirus
Storage / Stability of kits and reagents: 2-30 degrees for two years.
Accessories provided with Truenat Machine:
FOR SCREENING TEST for Beta CoV: Truenat RNA Extraction Machine.
- Cuvette Stand and Machine
- Cartridge Kit.
- Sample Chamber
- Elute Chamber
- Beta CoV kit
- Cuvette with RT enzyme.
- Micropipette.
- Beta CoV Chip
- Lysis buffer
CONFIRMATORY TEST for Sars CoV -2 Chip:
- SARS CoV-2 kit
- Cuvette with RT enzyme.
- Micropipette.
- Beta CoV Chip
- Amplification Machine and
- Thermal Printer
Note: Nucleic Acid Extraction should be done on Bio-safety cabinet level 2 or 3.
The Step-by-step procedure of micro-RT PCR with Truenat:
A. For Screening Test with Truenat:
To run a sample test in Truenat (Molbio diagnostic) machine, mainly two machines are used:
- Extraction machine
- Amplification machine
The following steps need to be followed:
1. Unpack the cartridge kit. Label the Cartridge with Patient id, Date, and time of test run. Also, label Elute collection tube.
2. Add 0.5 ml of VTM to lysis buffer through a provided dropper and then add 3ml of Mixed lysis buffer to the sample chamber of the cartridge. The used dropper and other used items should be discarded in 0.5% hypochlorite.
3. Close the cartridge chamber with provided black cap and insert the cartridge chamber in Amplification Machine and close it. Press the run button.
After 20 minutes, a beep sound means RNA extraction is complete. Transfer the cartridge to the cartridge stand, and now, we will prepare for the Amplification procedure in the next machine.
1. Open the elute chamber and transfer elute to the pre-labeled elute collection tube. Discard the cartridge to 0.5% Hypochlorite. After each step, sanitize your hands with a hand rub.
2. Transfer six microliters of eluting into the cuvette with RT enzyme and wait for 10-30 seconds.
3. Transfer elute to elute chamber in Beta CoV Chip and then load the chip with elute. Discard the tip of the Micropipette to hypochlorite.
4. Transfer Beta CoV Chip to amplification machine. (Before Transferring, patient details should be entered in the Amplification method.)
5. Similarly, another patient Beta CoV chip is transferred to the second chamber of the amplification machine.
After 40 minutes, a beep sound means the amplification test is complete.
For the screening test,i.e., Beta CoV comes positive then, the SARS Cov-2 chip has to be run for the Confirmatory test.
B. For the Confirmatory test following steps need to be followed:
- For the Sars Cov-2 test, we have to transfer the elute to Sars CoV-2 Chip and do amplification only.
- No extraction is required again.
- The same procedure of amplification is then repeated with the already extracted elute.
- After 40 cycles, the test will complete.
How to Read Result:
Beta CoV Chip detects E gene of Corona Virus (structural gene) screening test. At the end of the test run, Beta CoV “DETECTED” for Positive results or “NOT DETECTED” for Negative results is displayed on the screen.
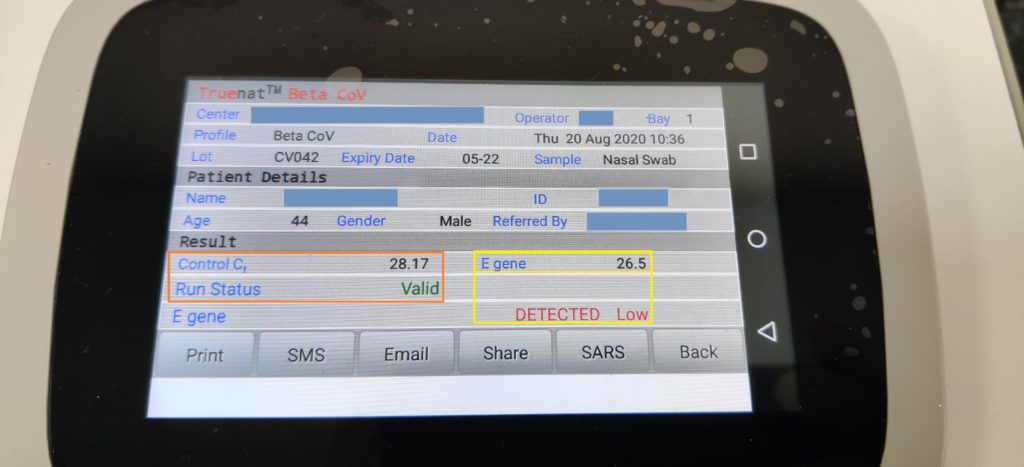
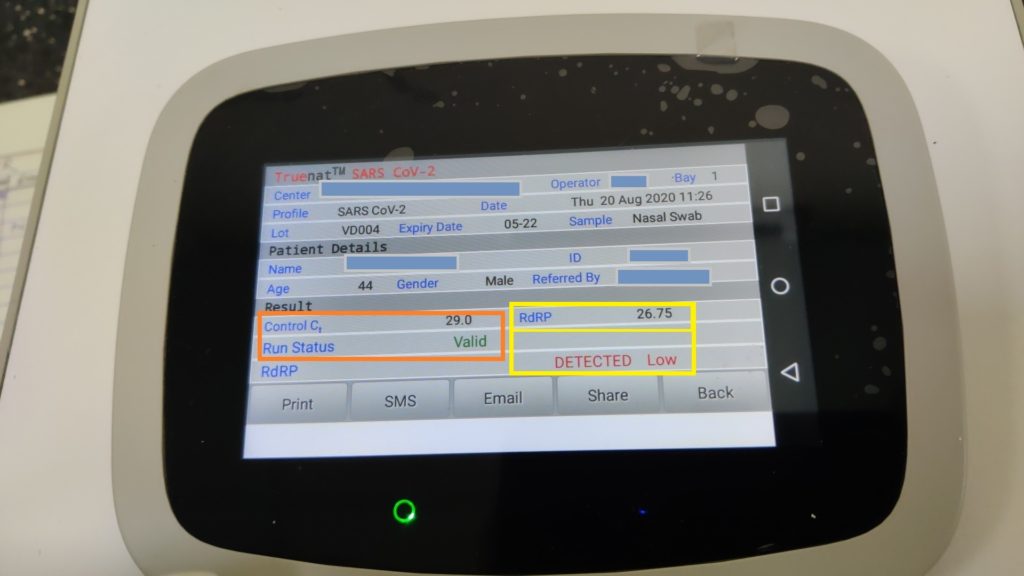
Sars Cov-2 Chip detects the RdRP gene of Coronavirus (Functional gene)- Confirmatory test. Similarly, At the end of the test run, SARS CoV-2 “DETECTED” for Positive result or “NOT DETECTED” for Negative result is displayed on the screen.
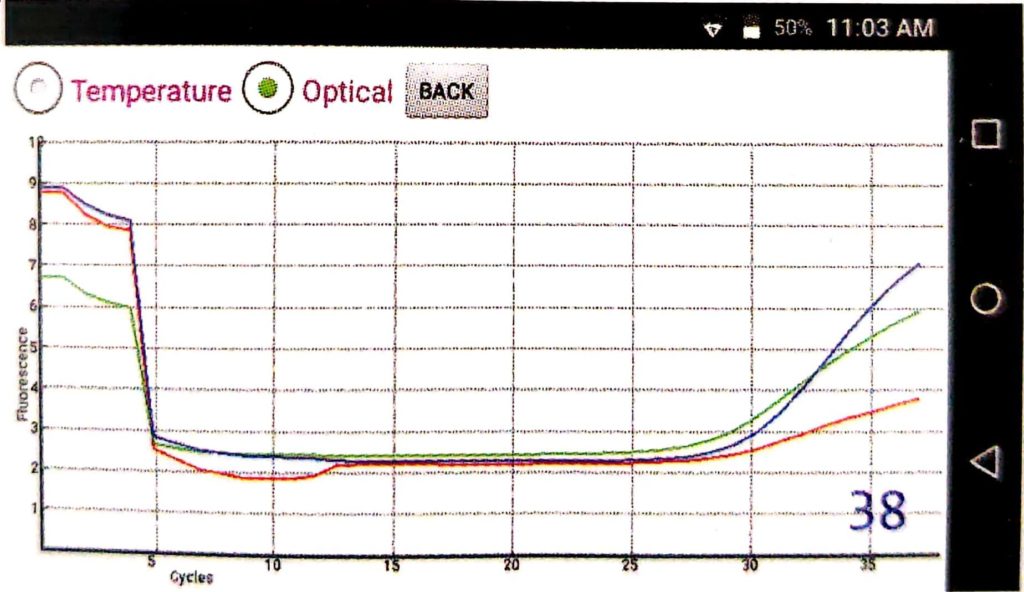
For Negative results, a horizontal amplification curve is displayed on the screen because amplification does not occur.
It is necessary to observe the Optical graph and Temperature graph after each test (Described in Figure 3 and 4, respectively)
Note: Based on the detection of the internal positive control (IPC), the validity of the test run is determined. The IPC is a standard positive control that undergoes all the processes the specimen undergoes – from extraction to amplification, thereby validating the test run from sample to result. Absence or shift of IPC and Ct beyond a pre-set range in case of negative samples Shows invalid results. While IPC will co-amplify in most positive cases but in any case, if IPC doesn’t amplify in samples having a high target load, the test run will be considered valid.
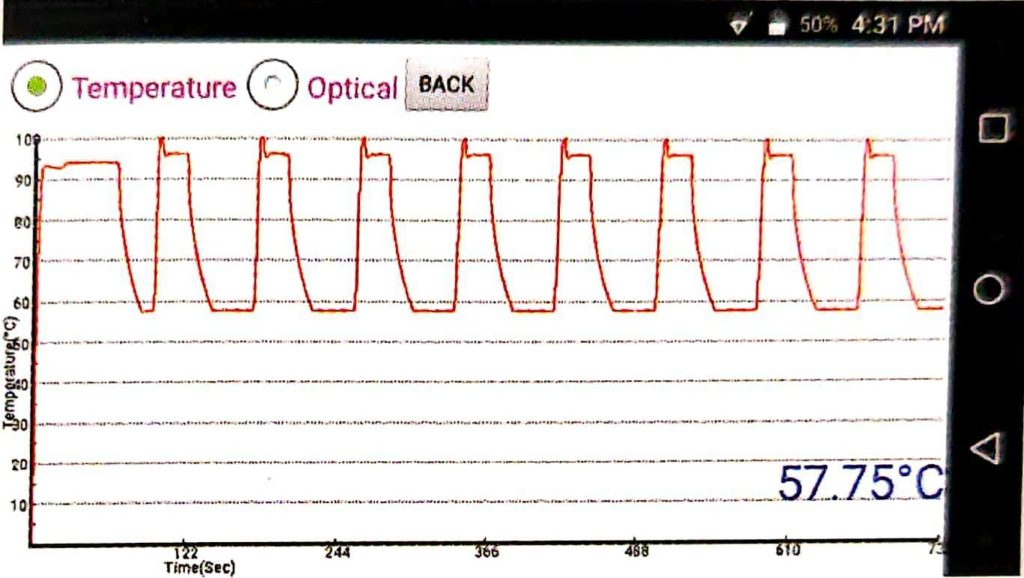
You can print the result provided with your Truenat machine. You can also transfer your truant data to the lab computer /or any remote computer via the internet. There is a capacity of Truenat to store data up to 20,000 results in the Amplification machine for future recall and references.
The notion of Ct value:
In almost any RT PCR, a positive result is detected by the accumulation of the final products of the replicative amplicon and hence the fluorescence signal. Response After PCR, every amplification curve is Defined with its cycle threshold (Ct) number.
The cycle threshold (Ct) is defined as the number of cycles required by the fluorescence signal to cross the threshold so that it exceeds the background signal of this machine.
Ct amounts are inversely proportional to the amount of target DNA (cDNA) of the sample. Thus, the lower the Ct level greater is the amount of target nucleic acid in the sample.
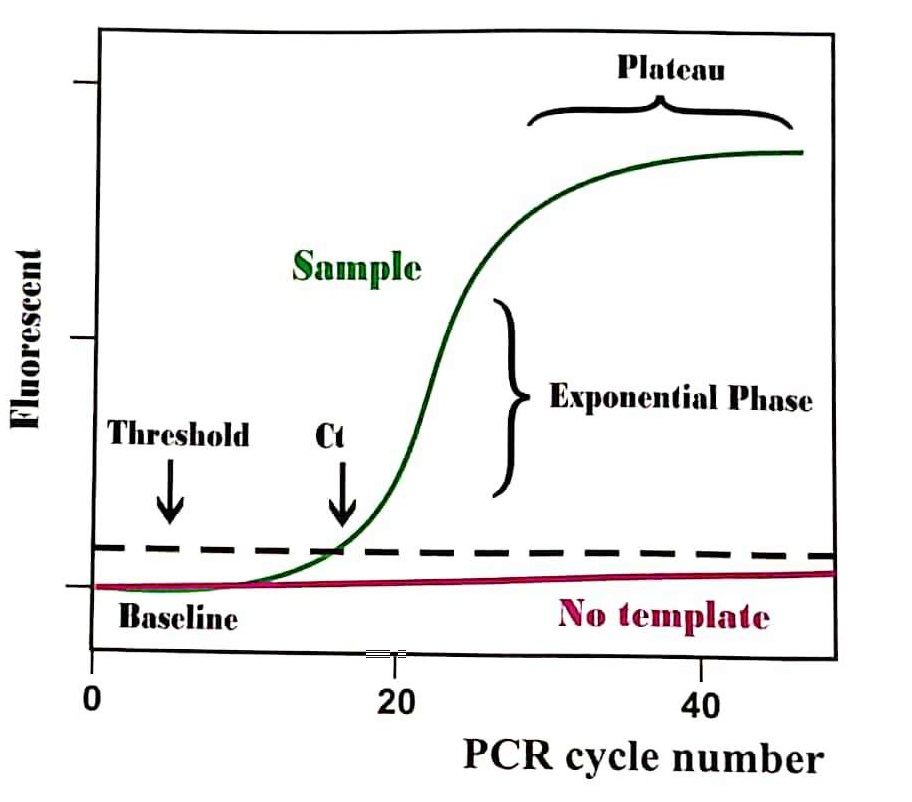
Ct value is Dependent upon many variables:
- Ct values might vary in different RTPCR machines: Different RTPCR machines have different Ct values as set by various companies. Their limitation of detection is different from 1 machine to a different machine.
- Sample collection time: If the sample is not taken correctly, it may limit the detection of the virus.
- Transportation of samples: If the sample is not transported to the laboratory as per instructions, it can hamper the quality of the outcome.
- The early or late phase of infection: In the early or late phase of infection, there is very less load of virus which might not be detected by RTPCR system and might yield higher Ct value and gives a negative result.
Note: Cycle threshold is a relative measure of the concentration of the target in the PCR reaction. Many factors decide the concentration of the target ten value of the Ct. Therefore, Ct values may vary according to the situation, and they may vary by using different PCR machines and/or reagents and hence, they should not be compared.
How to Interpret the Truenat result:
In this table you will see how to interpret the Truenat results.
| S.no. | Beta CoV test | SARS CoV-2 test | Test Status | Final result | Interpretation |
| 1. | Negative | – | Valid | Negative | Confirmed Negative |
| 2. | Positive | Positive | Valid | Positive | Confirmed Positive |
| 3. | Negative | – | Invalid | Invalid Test | The test is Invalid. Hence repeat. |
| 4. | Positive | Positive | Invalid | Invalid Test | The test is Invalid. Hence repeat. |
| 5. | Positive | Negative | Valid | Inconclusive | The test is Inconclusive. Repeat test after 2-3 days |
Advantages Of Truenat test:
- Relatively easier to perform the assay
- Need lesser space requirements to perform the test
- Relatively low human errors
Disadvantages of the Truenat test:
- Limited menu – Not cover the entire spectrum and also not able to update to better assay available
- It is supplier-dependent for supplies. Only Truenat cartridges and kits are used. No other company kits are used.
- High cost per test
- Low yield of the result. Only 1-2 tests/run.
- No multiplexing was seen.
Carry home message:
Truenat machines are Made in India products. They were widely used for Tuberculosis and Malaria testing. Nowadays, they are being used for Covid Testing also. So, it is important to know about the machine and how to use it. So, this article will help you in this quest.